Modern Methods of Extraction
Extraction can be defined as the removal of soluble chemical constituents from an insoluble liquid or solid material, by using or by the treatment of a liquid solvent.
It is therefore, the extraction is a solution process and depends on the mass transfer phenomena (transfer of chemical constituents from material to the solvent) in order to obtain improved yields of drug derived from plant and animal sources. Extraction involves the separation of medicinally active chemical constituents of plant or animal tissue using selective solvents in standard extraction procedures.Extraction involves the separation of medicinally active chemical constituents of plant or animal tissue using selective solvents in standard extraction procedures.
Essential steps of drug extraction
The process of drug extraction can summarily be divided into four essential steps:
- Penetration of the solvent into the drug
- Dissolution of constituents
- Outward diffusion of the solution from the cells
- Separation of dissolved portion and the exhausted drug
Traditional methods of extraction
The traditional methods of extraction are –
- Maceration
- Percolation
- Digestion
- Infusion
- Decoction
Modern methods used for phytochemical extraction are more efficient, sustainable, and more suitable for heat-sensitive compounds than traditional techniques. These modern methods offer reduced extraction times, lower solvent consumption, and improved yields of bioactive compounds and making them more cost-effective.
Modern extraction processes (overview)
Some of the modern processes of phytochemical extraction were explained here:
Supercritical Fluid Extraction (SFE)
Supercritical Fluid Extraction is a technique that uses a supercritical fluid (usually carbon dioxide, CO₂) as the solvent to extract desired compounds from plant or other materials.
A supercritical fluid is a substance that is neither a gas nor a liquid, but has properties of both. It forms when a substance is heated and pressurized above its critical temperature (Tc) and critical pressure (Pc). At that point:
- It behaves like a gas (can diffuse easily into materials)
- And like a liquid (can dissolve solutes effectively)
Its density is similar to that of a liquid, while its viscosity and diffusivity are similar to those of a gas. Therefore, a supercritical fluid can act as a solvent similar to a liquid, but with enhanced mass transfer kinetics.
A standard supercritical fluid extraction system consists of a high-pressure pump that provides the fluid and an extraction cell that holds the material, and is kept at the desired temperature and pressure.
Addition of co-solvents with CO₂
Supercritical CO₂ (scCO₂) behaves like a non-polar solvent (similar to hexane). Therefore, it easily dissolves non-polar or slightly polar compounds. Addition of ethanol, methanol, water, or isopropanol increases the polarity of the solvent system, allowing it to dissolve more polar molecules as well.
Typical SFE steps
- Pressurization: CO₂ is compressed and heated above its critical point → becomes supercritical. Then pumps used in this, compress the fluid (CO2) from a liquid to a supercritical state and deliver it at a precise, high pressure and flow rate into the extraction vessel. This is a critical step because the solvent's properties and extraction efficiency are directly dependent on pressure and temperature.
- Extraction: The supercritical CO₂ is passed through the plant material in the extraction vessel. It penetrates the plant matrix like a gas. It dissolves target compounds like a liquid. Then the mixture (extracted dissolved chemical constituent and CO₂) flows into a separator where pressure and temperature are reduced, due to this CO₂ returns to gas form and extracted compounds (solutes) precipitate out and are collected.
- CO₂ Recovery: Then the mixture (extracted dissolved chemical constituent and CO₂) flows into a separator where pressure and temperature are reduced, due to this CO₂ returns to gas form and extracted compounds (solutes) precipitate out and are collected. • CO₂ gas is recompressed and recycled in a closed loop system.
Advantage: The advantages of the process are lower viscosity and higher diffusion coefficient of super critical fluid than the normally used liquid solvent, and due to more penetration power to the super critical fluid, and higher mass transfer is achieved in less time duration of extraction, results in nearly complete extraction.
Drawback: Drawbacks of the process are the high initial cost of equipment, the high cost of operation, complicated process of extraction, and this process is labour-intensive.The most used solvent in supercritical state is carbon dioxide (CO₂).
Applications Supercritical fluids have been mainly applied for the extraction of valuable compounds from seeds, leaves, fruits, roots, flowers, rhizomes, and bark. This technique permits the extraction of different bioactive compounds including triglycerides, fatty acids, fatty alcohols, terpenoids, phytosterols, tocopherols, tocotrienols, and phenolics obtained from different plant matrices.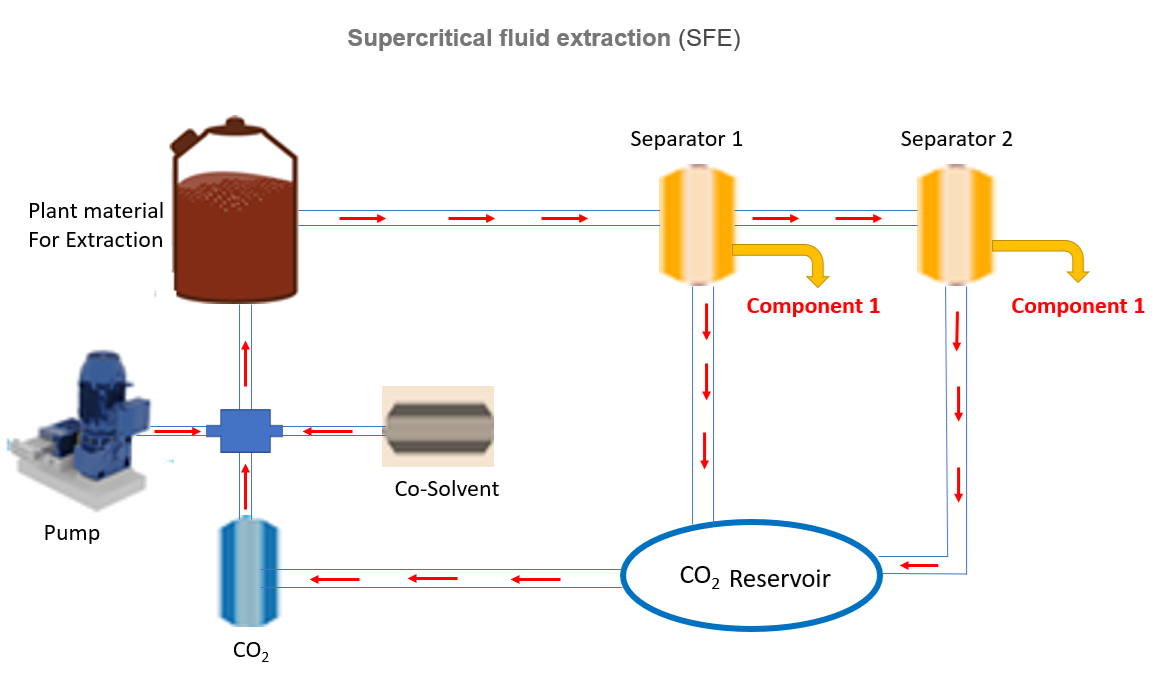
Ultrasonic-Assisted Extraction (UAE)
The majority of plant active components are primarily located within the vacuole of cell protoplasm. When a solvent enters the cell tissue, it dissolves these active components, which then transform into a leaching solution.
Unlike electromagnetic waves, ultrasonic waves are mechanical in nature and can propagate through solid, liquid, and gaseous mediums. UAE uses thermal, mechanical and the cavitation effect to extract bioactive components. During the action of ultrasonic, the cell wall is destroyed, while accelerating the release and diffusion of components in the cell. The thermal effect of ultrasonic waves refers to the phenomenon where the vibration energy of ultrasonic waves is absorbed by the medium and converted into heat. Consequently, the heat of the medium increases accordingly. The mechanical effect of ultrasonic refers to the phenomenon where the application of ultrasonic to a medium induces the vibration of particles within the medium in accordance with the mechanical wave. As a result, the motion of particles is enhanced, leading to an accelerated process of mass transfer. Ultrasonic cavitation means that under the influence of the ultrasonic, the minuscule bubbles (cavitation nuclei) within the liquid undergo vibrations, expansion, and violent collapse of microscopic bubbles in the extraction solvent. When these bubbles collapse near the plant cell walls:- They generate localized high temperature and pressure,
- Create shock waves and microjets,
- When bubbles collapse, they break plant cell walls, enhancing mass transfer of phytochemicals into the solvent.
- After extraction, the solution is filtered and concentrated to obtain the extract.
Accelerated Solvent Extraction (ASE)
Accelerated Solvent Extraction is a modern technique used to extract phytochemicals from plant material by using organic solvents at elevated temperature and pressure.
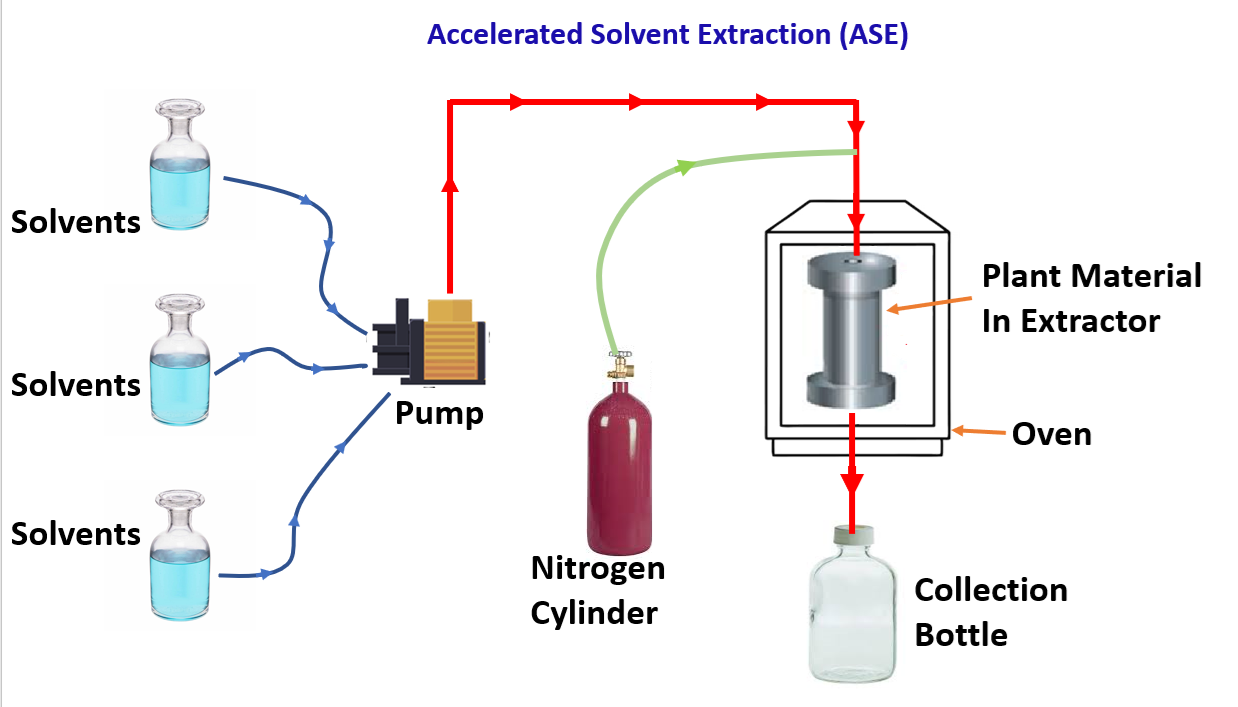
This method is called the Accelerated solvent extraction because in this process, increase in the temperature and pressure of the solvent above its boiling point enhances the solvent’s ability to dissolve analytes and accelerates the extraction process. Moreover, higher temperature reduces viscosity, which means that solvent can penetrate the pores of the matrix more easily.Main components of ASE apparatus:
- Solvent reservoir
- Pump (to force solvent into extraction cell under pressure)
- Extraction cell (contains packed solid sample)
- Oven (heats cell to desired temperature)
- Collection vial (collects extracted solution)
- Nitrogen gas supply (flushes system after extraction)
In this technique, the extraction cell made of stainless steel is packed with the sample, then filled with solvent, and placed between inert silica layers separated with cellulosic filter papers.
The system is heated at an increased temperature and pressure for a preset time, the conditions favour extraction due to increased diffusion coefficient and lowered viscosity. The extract is collected in vials and cell cleaned by pumping fresh solvent and nitrogen. The major Advantages of this process is Rapid extraction (minutes instead of hours), Uses less solvent, Automated and reproducible and High efficiency and recovery of phytoconsituents. But this process is not suitable for thermolabile compounds.When extraction is complete, compressed nitrogen moves solvent from the cell to the collection vial. The filtered extract is ready for analysis.
Summary
Modern extraction techniques such as supercritical fluid extraction, ultrasonic-assisted extraction and accelerated solvent extraction improve extraction efficiency, reduce solvent usage, and cut processing time — while being more suitable for heat-sensitive phytochemicals when properly optimized. Each method has its own advantages and limitations that should be considered based on the target compounds, matrix properties, and scale of operation.