Preformulation Studies
Definition
Preformulation testing is the first step for the rational development of dosage forms of a drug substance. Preformulation studies are the investigations of physical and chemical properties of a drug substance, alone and in combination with other excipients.
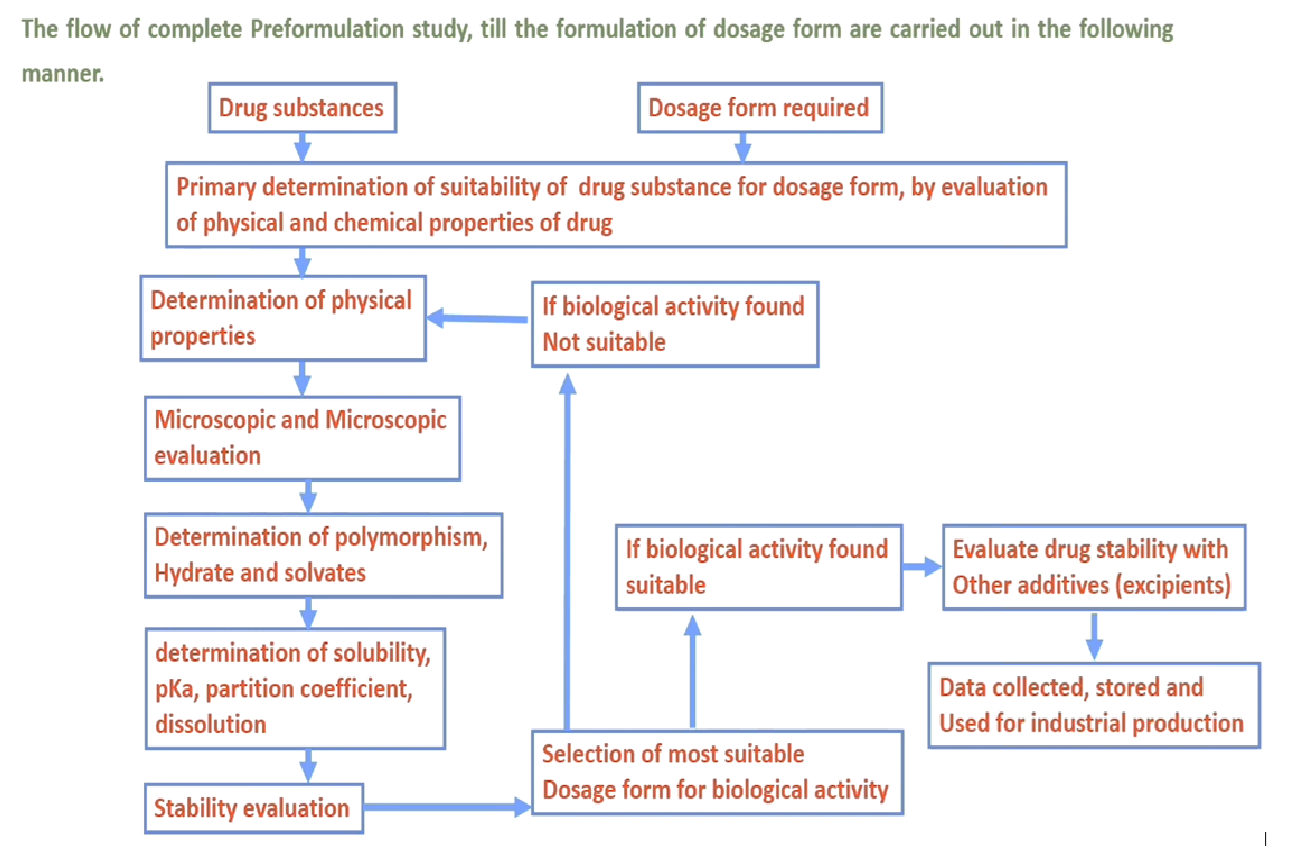
“Preformulation may be defines as the process of optimizing a drug through the determination of those physical and chemical properties, which are considered important for the formulation of safe, effective and stable dosage form”. Preformulation studies mainly performed according to the dosage form to be develop. The flow of complete Preformulation studies, till formulation of the dosage form is carried out in following manner.Objectives of Preformulation Studies
- The basic objectives of Preformulation studies are:
- To establish the physicochemical parameters of a drug molecule for its suitability for any desired dosage form.
- To determine the kinetic rate profile of drug substance for drug stability.
- To establish compatibility of any drug with formulation excipients for any possible interaction.
- Preparation of safe, effective and stable dosage form for better therapeutic values.
Physical Considerations of Preformulation
Physical Form (Crystalline or Amorphous):
- This is an important preformulation consideration because many drugs (although chemically stable) may exist in more than one crystalline lattice form or crystalline structure. Due to this these drugs may differ in melting points, solubility, density, vapour pressure and compressibility. This happens because each crystal molecule arrangement has different energy value.
- Suspension stability and syringeability – crystalline Penicillin-G is more stable than amorphous. Plate shaped crystals will flow through a needle more easily as compared to needle shaped crystals.
- Tabletting properties – these may be altered due to crystal packing arrangements during compression.
- Dissolution – crystal structure affects their surface area available for contact, which affect the dissolution rate property of any dosage form.
Crystalline habits may influence some properties:
Amorphous drugs are those substances that don’t have any defined molecular arrangements or defined molecular structure. These drugs generally have higher solubility and less stability.
Crystals are characterized by repeated arrangements of atoms or molecules in three-dimensional array, whereas amorphous forms have atoms or molecules randomly placed.Polymorphism:
- Polymorphism is the ability of a solid material to exist in more than one form of crystal structure. Chemical stability and solubility change due to polymorphism, which further affect the drug bioavailability. Polymorph can be classified as enantiotropic (reversibly change into another form at varying temperature or pressure) and monotropic (unstable at all temperatures). During preformulation, it is important to identify if the polymorph is stable at room temperature or not, to further determine the stability of drug formulation. Polymorphs are also evaluated during preformulation studies to check whether compounds withstand drying and milling during product formulation.
Particle Size:
- Particle size and size distribution affect may properties of drug and formulations, i.e. drug dissolution rate, absorption rate, content uniformity, taste, texture, colour and stability.
Size optimization is considered important for both. i.e. for drug and formulation additive mixing and content uniformity in any dosage form.
Optimized formulation requires controlled size reduction. Particle size reduction less than 10μ, cause many problems like incorporation of gas / air between particles, which prevents the wetting of drug particles.
Extreme size reduction, produces electrical charges over particles, that adversely affect formulation and drug stability.
Some extreme size reduction increases surface area, which may cause polymorphic changes, rapid degradation, when exposed to heat, light, humidity and air.
Common methods for size analysis in industry are microscopy, sieving, coulter counter, laser diffraction method etc.
Partition Coefficient:
- Partition coefficient is an important preformulation consideration which determine the absorption of any drug through biological membrane (which is mainly composed of lipids).
As every drug has to cross this lipid membrane before absorption, thus trough partition coefficient determination. The drug distribution property between organic solvent and water is called the partition coefficient. This is calculated by the formula:

- When a solute which is soluble in each of these two solvents, gets distributed between these two phases and the ration of drug distributed between these two phases is called partition coefficient.
Generally higher partition coefficient shows better absorption and better bioavailability.
pKa:
- This is also called drug dissociation constant. pKa determination provides information about drugs ionization at any pH. This parameter is determining the absorption of any drug to biological tissue. It helps predict how molecules will behave in acid-base reactions.
pKa is a measure of how easily a compound gives up a proton (H⁺) — essentially, it's a number that tells you how acidic a molecule is
pKa=−log10(Ka)
where (Ka) is the acid dissociation constant.
low pKa means a strong acid (it gives up its proton easily).
high pKa means a weak acid (it holds onto its proton more tightly).
Solubility Profile:
- Drugs solubility profile is important consideration which greatly influence the drug absorption and its bioavailability. Various drugs of limited solubility may cause some problems of poor absorption, drug accumulation and related toxicity due to higher dose. Preformulation techniques are used enhance the solubility and minimize these problems. The solubility can be enhanced by making salts of drugs, or by making other soluble derivatives.
Some other techniques to improve solubility are complexation, micronization, solid dispersion etc.
In some cases, solubility gets reduced to improve taste (in case of clindamycin ester) and also to reduce the solubility to make a dosage form sustain release to get prolong effect.
Chemical Considerations of Preformulation
Hydrolysis:
- Hydrolysis is the chemical breakdown of a compound, due to reaction with water. H+ and OH- are the reactive species, responsible for drug hydrolysis in a solution. Hydroxyl ions are stronger nucleophiles than water. Thus, degradation reaction is higher in alkaline solution than in water.
In solutions of low pH, H+ ion causes hydrolysis reactions. Hydrolysis is mainly considered in preformulation studies of solutions and specially deals with drug stability and drug degradation.
Hydrolysis is affected by pH of the solution or ionic strength, presence of buffer salts, presence of cosolvents, complexing agents and surfactants.
Oxidation:
- The second most common way a compound can decompose in solution is via oxidation. Oxidation is promoted by the presence of oxygen and the reaction can be initiated by the action of heat, light and trace metal ions that produce organic free radicles. These radicles propagate or enhance the oxidation reaction that ultimately degrade the compound or any drug molecule.
Racemization:
- Racemization is an preformulation consideration because during formulation development, it has to be ensure that the racemization should not occur under the labelled conditions for storage and use within the proposed shelf life.
Simply racemization is a conversation (by heat or by any chemical reaction) of an optically active compound into an optically inactive form.
Racemization is a common mechanism of degradation, resulting in loss of biological activity of drugs.