HLB Scale
Emulsions are dispersed systems made of the mixture of two immiscible liquid phases which is stabilized by the surfactants.
The knowledge about hydrophilic-lipophilic balance (HLB) is an essential parameter related to the emulsion production because, it allows the selection of a suitable surfactant or even a surfactant blend which is required to produce a stable emulsified system.The concept of HLB was originally described by Griffin and later refined by Davies. Griffin’s HLB System was an attempt to systemize the choice of a suitable surfactant.
According to which, surfactants and emulsifiers are classified in terms of HLB values required to produce a stable emulsified system.Surfactant molecules have both hydrophilic (water loving) and lipophilic (oil loving) groups. The HLB system stands for “Hydrophilic Lipophilic Balance”.
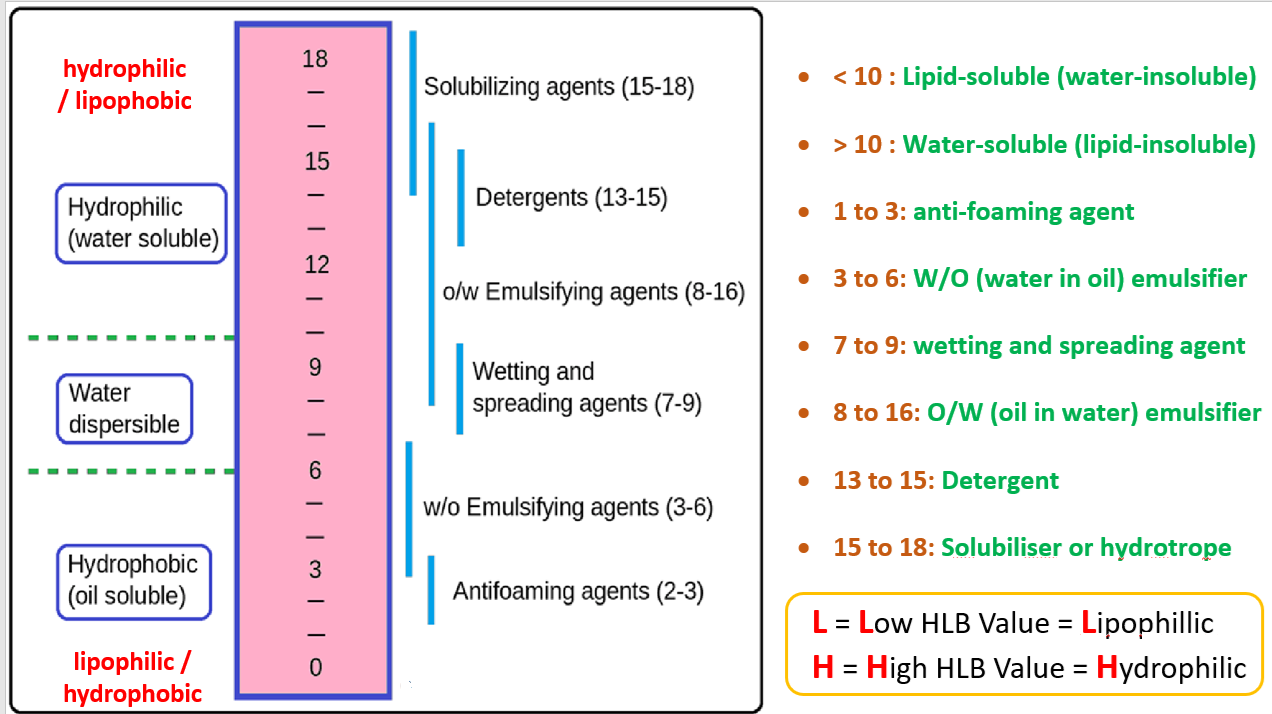
Hydrophilic–lipophilic balance (HLB) on a scale of 0 to 20, is the balance of the size and strength of the hydrophilic and lipophilic moieties of a surfactant molecule.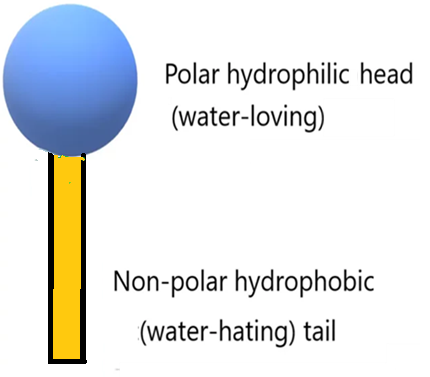
Thus, each surfactant presents one intrinsic number (its HLB value), which represents its hydrophilic / lipophilic property.
An HLB value of 0 corresponds to a completely lipophilic / hydrophobic molecule, while a value of 20 corresponds to a completely hydrophilic / lipophobic molecule.The scale helps formulators to choose the right surfactant for making stable emulsions, detergents, wetting agents, and solubilizers.
In short, the HLB system predicts whether a surfactant will behave better with the water or with the oil.HLB Value – Reason to Select Surfactant / Emulsifier
Which HLB value Emulsifying agent works well in oil-in-water (O/W) emulsions?
The agent is selected according to continuous phase. Here the continuous phase is water and dispersed phase is oil (O/W), hence the suitable agent will be water loving means high HLB value.
Which HLB value emulsifying agent works better in water-in-oil (W/O) emulsions?
In water-in-oil (W/O) emulsion, water is dispersed phase and oil is continuous phase, hence the emulsifying agent should be soluble in oil phase—means lipophilic, having low HLB value.
Basic Theory for Emulsification
In an emulsion system, the emulsifier must always be more soluble in the continuous phase and less soluble in the dispersed phase, because this solubility pattern determines where the emulsifier will position itself. When an emulsifier is highly soluble in the continuous phase, it distributes throughout that phase and migrates to the interface where dispersed droplets are present. At this interface, the emulsifier molecules forms a stable, protective film around the droplets of the dispersed phase. This film prevents the droplets from coming together and coalescing, thereby improving the stability of the emulsion.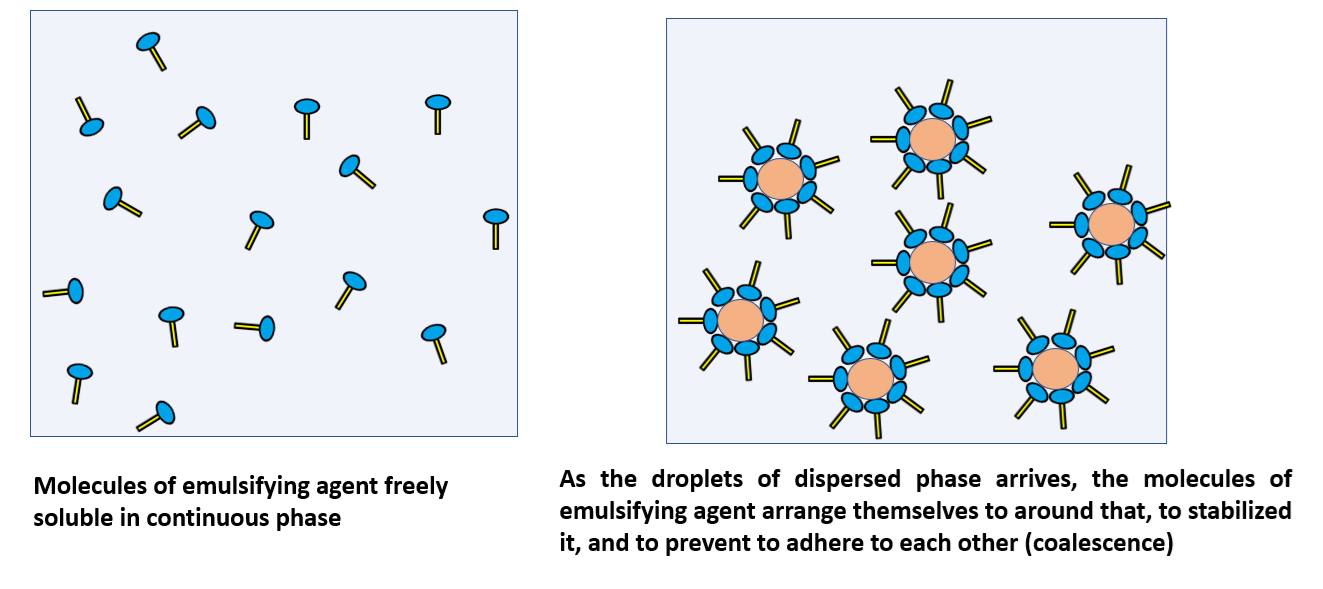
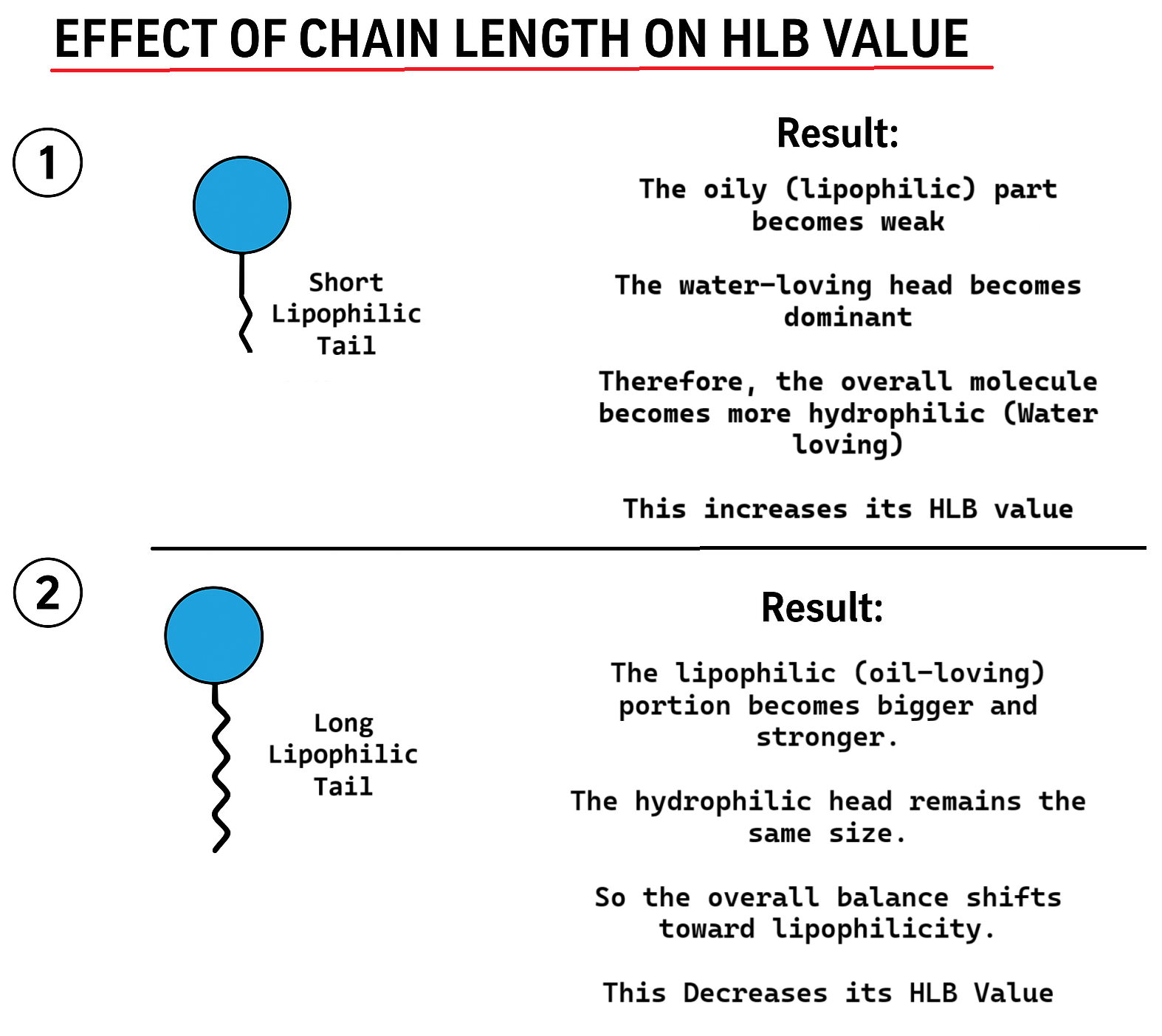
Role of hydrocarbon Chain length on HLB Value
The length of the hydrocarbon chain in a surfactant strongly influences its HLB value, because the chain determines the lipophilicity (oil-loving) and Hydrophilicity (water-loving) property of the molecule. When the hydrophobic chain becomes longer, the surfactant becomes more oil-soluble and less water-soluble, causing its HLB value to decrease. In contrast, if the hydrophobic chain is shorter, the molecule becomes relatively more hydrophilic compared to its lipophilic part, which increases the HLB value. Therefore, increasing chain length shifts the balance toward lipophilicity, while decreasing chain length increases hydrophilicity.