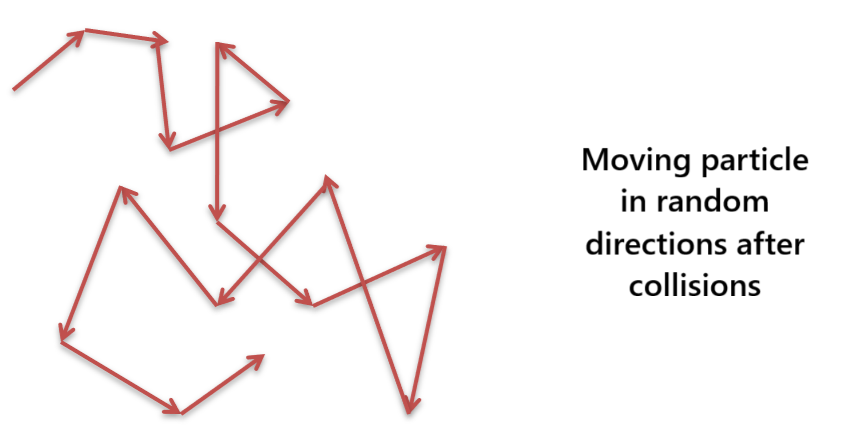
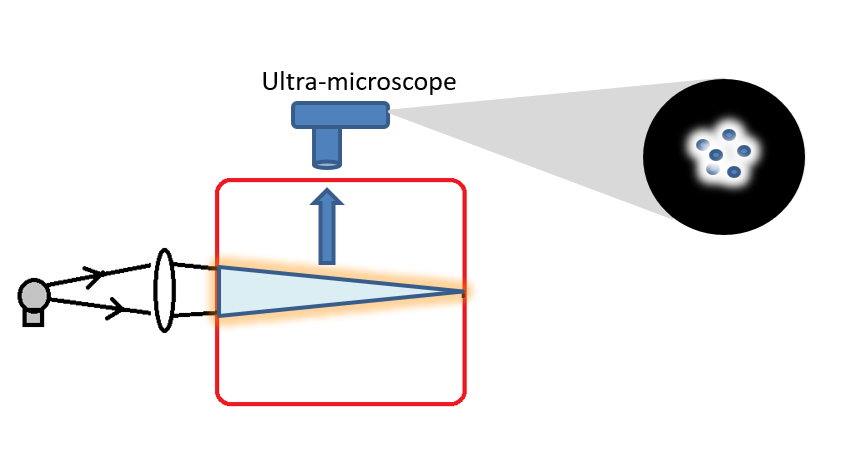
When a colloidal solution is viewed under an ultra microscope, the colloidal particles are seen continuously moving in a zigzag path. Brownian movement is due to the unequal bombardments of the moving molecules of the dispersion medium on colloidal particles. The moving molecules of the dispersion medium continuously attack on colloidal particles from all sides and impart momentum to them.
It is more rapid when the size of the particles is small and the solution is less viscous. Since the chances of their collisions are unequal, the net driving force on a colloidal particle forces it to move a particular direction. As the particle moves in that direction, other molecules of the medium again collide with it and the particle changes its direction. The process continues. This results in a random zigzag movement of the colloidal particle.